ABSTRACT
The two most important factors for determining the risk of local failure and overall prognosis in colorectal carcinoma are nodal status and the depth of tumor penetration into or through the bowel wall. These features have traditionally been determined pathologically because the clinical-staging accuracy of other imaging modalities such as computed tomography (CT) has not proven sufficiently predictive of surgical staging. However, endorectal or endoscopic ultrasonography (EUS) can be used to preoperatively evaluate nodal involvement with an accuracy of up to 86% (median: 80%) and depth of tumor penetration through the bowel wall with an accuracy of up to 97% (median: 85%) for effective clinical staging. This high staging accuracy is useful in managing colorectal cancer. Through clinical evaluation of the initial stage of colorectal cancer with EUS, a patient's risk of disease recurrence can best be determined and patients stratified for the most appropriate treatment. EUS can be used to select patients with lesions that can be treated with local excision or sphincter-sparing surgery, often combined with radiation therapy, in situations otherwise requiring an abdominoperineal resection. EUS can also be used to preoperatively identify patients with locally advanced or unresectable disease. Chemoradiation can then be given preoperatively, when it appears to be better, tolerated and more effective than postoperative treatment. Unresectable tumors can often be downstaged sufficiently to allow their excision. In resectable disease, EUS can also identify patients at high risk for recurrence who would benefit from adjuvant chemoirradiation. EUS for precise staging or for earlier diagnosis of recurrence will further improve the clinical outcome of patients with colorectal tumors as significant advances both in surgical techniques and in combined chemotherapy/radiotherapy continue to be made and applied selectively in a stage-dependent manner.
INTRODUCTION
Echoendoscopy or endoscopic ultrasonography (EUS) has been the most significant advance in the past decade for imaging the gastrointestinal (Gl)-tract wall and contiguous organs (1). The detailed resolution of the images provided by EUS is unmatched by any other current imaging method. However, the role of EUS in the management and outcome of patients with neoplastic disease is clearly dependent upon both its effect on accurate staging and the availability of effective treatment strategies. Many studies document the high accuracy that is possible with EUS in the nonoperative characterization of GI tumors and in tumor-node-metastasis (TNM) staging (1). EUS can provide information essential for diagnosing malignancy that is often otherwise obtainable only by surgery. Although rectal cancer has traditionally been staged pathologically with surgery, highly accurate colorectal tumor staging is now possible without surgery through imaging with endorectal ultrasonography (RUS) or EUS.
For patients who remain at high risk of recurrence despite radical surgical excision of rectal carcinoma, combined chemotherapy/radiotherapy has been the standard of care since 1991 (2,3). Multiple randomized trials formed the basis of a National Institutes of Health (NIH) Consensus Development Conference and a National Cancer Institute (NCI) Clinical Announcement recommending postoperative radiation therapy combined with 5-fluorouracil-based chemotherapy for these high-risk patients because it "can reduce overall tumor recurrence rates, substantially reduce local recurrence, and prolong patient survival." Preoperative chemotherapy plus radiotherapy has also been studied for rectal carcinoma (4-22), and appears more effective and better tolerated than postoperative chemoradiotherapy.
The TNM system has proven extremely valuable for staging and determining prognosis in carcinoma. Patients without metastatic disease to regional lymph nodes, and whose rectosigmoid tumors have not penetrated through the bowel wall (TNM stage = T1-2N0 M0), have an 80% or better overall survival and less than 10% local failure after resection. Tumors such as the one illustrated in Figure 1, which have breached the colorectal wall (T3-4 N0M0 or MAC stage B2, B3) or spread to lymph nodes (T1-4 N1-3 M0 or MAC stage C2, C3), have the highest risk of recurrence. In these patients, local failure increases to 25-50% and 5-year survival decreases to only 40-50%.
In this review, methods of preoperative staging for stratifying the treatment of colorectal neoplasms are described and compared. The role of RUS/EUS in planning combinations of chemotherapy, radiotherapy, and surgical treatment is reviewed. The synergistic advantages of accurate RUS/EUS and better-tolerated preoperative chemoradiotherapy are discussed.
METHODS AND EQUIPMENT
RUS can be performed with blind probes or echoendoscopes. Patient preparation for the two types of examination is similar. However, the technique differs in that during EUS with the echoendoscope, direct endoscopic visualization can aid in localizing the lesion. Also, the echoendoscope can be inserted, depending on the operator's expertise, far beyond the rectum, whereas during RUS the blind probe is limited to the distal rectosigmoid region.
Much of the early clinical experience with RUS has been with radial scanning, rigid probes that have an attached transducer connected to a hand-held motor unit. This transducer transmits the ultrasound waves at a 90-degree angle to the axis of the insertion rod, rotates at 406 cycles per second (cps), and usually uses a 7.0-MHz frequency. After rectal-cleansing enemas, the probe is inserted into the rectum directly or through a rigid proctoscope, with the patient in the left lateral decubitus position. Inflating a thin balloon or sheath fitted over the transducer with 60 ml of deaerated water, or using a water-soluble gel between the sheath and transducer, achieves adequate acoustical coupling. To fully insert the rigid probe past the tumor, the rectal lumen must have a minimum diameter of 2.5 cm.
The rectum can also be examined with a flexible, oblique-viewing echoendoscope with either a 360-degree radial scanning transducer or a linear curved-array transducer at the tip of the instrument. The echoendoscope has a tip diameter of up to 12.6 mm, with a 4.2 cm rigid tip, and scanning frequencies of 7.5 and 12.0 MHz. After full insertion of the instrument into the rectum and colon, filling of the transducer balloon with water or instillation of water directly into the rectal lumen provides the required acoustical interface for the examination. The echoendoscope is then gradually withdrawn while the controls are used to manipulate the transducer into the best position for examining the target area. Both 7.5 - and 12.0-MHz frequencies are typically used during the examination. Imaging of adjacent structures, such as bladder, seminal vesicles, prostate, and vagina with lower frequencies aids orientation, while the 12.0-MHz frequency provides a more defined view of the rectal wall.
Ultrasound probes with a frequency range of 15-50 MHz are being developed but have not been extensively evaluated. These high-frequency probes may prove clinically valuable for the evaluation of superficial lesions (23), but not for deeper lesions. The probes pass through standard endoscopes and can be placed directly against lesions not easily imaged with other instruments.
ACCURATE TNM STAGING
Many studies and reviews have followed Dragstedt and Gammelgard's initial report of RUS for the staging of rectal cancer (24). Median estimates of the accuracy of RUS as compared with computed tomography (CT) from over 25 studies, summarized in a recent review (1), were 85% versus 70%, respectively, for T stage, and 80% versus 55%, respectively, for N stage. For preoperative staging of rectal cancer, the accuracy of RUS is significantly superior to that of either CT or digital examination (DE) because it can accurately predict depth of invasion on a layer-by-layer basis in the rectal wall.
Early experience with lower-frequency (5.5 MHz) rigid probes demonstrated that higher frequencies were necessary for adequate lymph-node staging. Subsequent comparative studies, using frequencies of 7.0 MHz or greater, have shown that both rigid ultrasound probes and radial or linear echoendoscopes provide an accuracy superior to that of CT for both T and N staging of colorectal carcinoma (1).
Magnetic resonance imaging (MRI) does not offer any significant staging advantage over CT, since similar problems are encountered in assessing rectal-wall extension of disease and lymphadenopathy (25,26), resulting in similar values for accuracy. In a prospective comparative study of preoperative staging of rectal cancer, the respective accuracies of T stage with RUS and MRI were 88% and 82%, and the respective accuracies for N stage were 80% and 60% (27). MRI rectal-coil probes have been evaluated for locoregional staging. In the limited number of studies so far conducted, correct staging was found in 67% of patients, with an accuracy of 79% for T stage and 65% for N stage (28,29). Although the rectal-coil probes provide more detailed images of the layers of the rectal wall than does standard MRI, their clinical utility has not been defined, and no benefit for them has been demonstrated as compared to RUS.
CT is necessary for evaluating patients with suspected distant disease, such as metastatic spread to the liver. However, even for this purpose, its sensitivity is less than 75%, because peritoneal disease or liver metastases smaller than 2 cm are frequently not detected for many GI tumors (1). In our opinion, the literature supports a combination of RUS or EUS for T and N stages and CT for M stage as the most clinically useful approach for comprehensive staging of rectal carcinoma.
T Stage
Visual inspection and digital palpation were used to clinically stage rectal cancer prior to the development of CT (30-36). For palpable rectal lesions, a digital examination (DE) by an experienced examiner can give a staging accuracy of 60-80%. A digital rectal examination therefore sets a high level of accuracy for low lesions that, prior to the development of EUS, had proven difficult to improve upon even with extensive technological evaluation (37,38). Nevertheless, there are limitations to DE. In one-third of rectal cancers, the tumor is too proximal to be palpated. DE also tends to be less accurate for early-stage lesions confined to the bowel wall than for more advanced disease. Accuracy does not improve even if the rectal examination is done with the patient under general anesthesia (33). In attempts to improve the accuracy of staging of local disease, various other imaging modalities have been tried.
Many techniques and preparations have been used to maximize the accuracy of CT in evaluating the local spread of rectal cancer (39,40). Despite attempts to define criteria for CT staging, CT is limited in its ability to accurately stage rectal cancer, owing to its lack of definition of the degree of mural involvement and its low sensitivity for malignant lymph nodes (41-44). As an example of this, the advanced T4 N 1 M0 tumor shown in Figure 1 was incorrectly staged by CT as T2 N 0 M0. CT identified rectal-wall thickness, but no invasion or diseased nodes.
Figure 1a
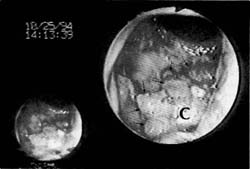
Figure 1b
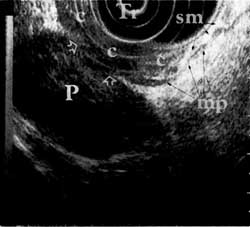
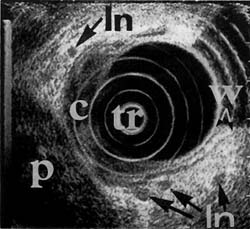
Figure 1c
Only one study could be found that directly compared DE, CT, and EUS for accuracy of local staging in the same patients with palpable rectal adenocarcinomas. The respective accuracies were 62%, 71%, and 95% (33). CT scanning therefore does not significantly improve the accuracy of local staging even as compared with DE unless the lesion is not palpable.
Few studies have addressed the influence of clinical information and interobserver variability in the use of RUS. Roubein et al. (45), recently examined these issues in a prospective blinded study, and found that flexible sigmoidoscopy plus DE accurately predicted whether a tumor had invaded through the rectal wall (T3) in 71 % of examined tumors. RUS accurately predicted T3 tumors in up to 96% of the same patients, with 88% overall agreement between two different endoscopists for T stage. In these same patients, there was 73% agreement in lymph-node staging. Knowledge of the patient's history or findings on physical examination, or of endoscopic visual inspection of the lesion, did not influence the RUS interpretation.
The accuracy of RUS in revealing depth of invasion of rectal carcinoma is at least 94% for T1, 73% for T2, 92% for T3, and 94% for T4 disease (1). T2 lesions are the most difficult to stage accurately with EUS because inflammatory changes can make a lesion invading the muscularis propria (T2) appear as if it is just breaking through into the adventitia (a T3 lesion) (46-48). In one study, T2 overstaging resulted in only a 24% specificity of staging (49). A prospective study of RUS showed an overall accuracy of 89%, with overstaging in 10.2% and understaging in 0.8% of cases. Staging was incorrect in 16.7% of lower-rectal tumors and 6.7% of upper-rectal tumors (50). Linear electronic intrarectal ultrasonography for staging of cancer of the rectum was studied in 45 patients (50). The accuracy of the linear curved-array transducer proved similar to that of the sector-scan instrument. Its accuracy was 82% for overall stage, 93% for T stage, and 84% for N stage as compared to histological and surgical findings.
N Stage
Detection of lymph-node metastases with CT is based on size criterion alone. Lymph nodes exceeding 1 cm in size are considered abnormal (1,40). The limited accuracy of CT for the detection of involved lymph nodes is due to insufficient spatial resolution and the relatively common occurrence of metastasis in lymph nodes measuring less than 1 cm in size (1,43,51,52). Unlike CT, EUS does not depend on size as the only criterion for determining whether a lymph node is malignant (1). Normal, nonenlarged lymph nodes are similar in echogenicity to the hyperechoic perirectal tissues, and therefore are not usually seen. Enlarged inflammatory lymph nodes are also usually hyperechoic, with ill-defined margins. In contrast, malignant lymph nodes are usually round, with smooth, sharply defined margins, and echogenic characteristics similar to those of the primary tumor, which is usually hypoechoic. Even though blood vessels can simulate malignant nodes, they can be differentiated by moving the transducer to outline the linear course of the vessel. A mesenteric implant, confirmed at surgery, can also resemble a lymph node (Fig. 2), the only difference appearing to be the irregular border of the lesion. Although RUS has allowed significant improvement in the accuracy of evaluation of N stage, diagnostic problems still exist because more than two-thirds of malignant lymph nodes are smaller than 5 mm (53).RUS can detect nodes as small as 3 mm, but endosonographers who depend on size as the exclusive criterion for
malignancy can reduce the sensitivity of the technique.
Although RUS is almost twice as sensitive as CT, it may, with the use of current criteria, still reveal only about half of malignant nodes (43). Nevertheless, the overall accuracy of RUS for local staging of rectal carcinoma (1) is at least 79% for stage N0(non-neoplastic nodes) and 74% for N1 (nodes positive for cancer). Preoperative biopsy of perirectal lymph nodes in rectal cancer with the use of RUS has also been evaluated, with one study obtaining an accuracy of 77% (54). Fine-needle aspiration (FNA) may improve the accuracy of lymph-node assessment. A more quantitative method for lymph-node differentiation may be necessary to improve the accuracy of N staging in the future (55).
M Stage
CT is commonly used to evaluate whether distant metastases are present in patients with colorectal cancer. The median accuracy for detection of metastases by CT is more than 75% (1). The relatively high level of accuracy of CT is positively influenced by the low prevalence of distant disease at the time of diagnosis of colorectal cancer. However, detection of peritoneal disease or liver metastases smaller than 2 cm with CT is limited. EUS can detect regional peritoneal disease (Fig 2). EUS can also reveal metastatic disease in the liver (1). However, because the liver can be evaluated only by oral insertion of the echoendoscope, EUS is in our opinion impractical and not cost-effective for detecting distant spread of colorectal carcinoma , and is unlikely to change the outcome in cases of such diseases.
Figure 2a
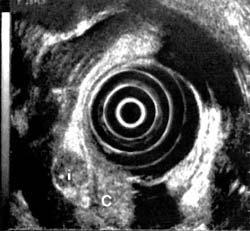
Figure 2a

EVALUATING TUMOR RECURRENCE WITH EUS
EUS accurately identifies the recurrence of colorectal tumors (56) in a manner similar to the identification with EUS of anastomotic recurrences of resected esophago-gastric lesions. When recurrence is suspected at a site of prior resection, early diagnosis can be made with standard superficial mucosal biopsy in only one-third of recurrences involving the mucosa. Extraluminal recurrence that eventually breaks through the mucosa is more common than endoscopically visible superficial recurrence at an anastomotic suture line. CT cannot reliably differentiate postoperative changes from recurrent tumor until the latter is well advanced. Therefore, RUS is essential in these cases, and allows directed deep biopsies of suspicious areas in order to confirm the recurrence of carcinoma not involving superficial mucosa.
Beynon et al. (57) reported findings for 85 patients who were screened postoperatively through clinical examination, sigmoidoscopy, and RUS. Recurrence was documented in 22 of the patients, in 19 of whom it was demonstrated by sigmoidoscopy/digital examination and in an additional 3 by RUS alone. Another study found that RUS revealed disease recurrence in 13 of 66 patients, but in 10 it could also be detected just through DE and rigid sigmoidoscopy. RUS therefore improves the ability to diagnose recurrent neoplasm by as much as 30% (58).
Local recurrence occurs in 20-30% of resected colorectal tumors (57-61). In a study of recurrence patterns in rectal carcinoma, Barras et al. (62) found that local recurrence correlated with T stage, the lowest rate being 10% for T2 lesions. Five-year survival for patients with spread to regional lymph nodes was 60%, but among those with spread to distant nodes, there were no long-term survivors. Overall 5-year survival was 25% when N stage was positive, with an associated 64% risk for metastatic disease in these patients.
Routine RUS in the follow-up of patients treated for rectal cancers could reveal early recurrences at a stage at which surgical treatment remains an option. Clinical and endoscopic assessment, followed by selective RUS, appears effective in the postoperative follow-up of patients with resected rectal cancer. Further studies are necessary to determine the optimal timing and interval for postoperative endosonographic assessment and to establish improved criteria for differentiating fibrosis from tumor after radiotherapy.
COLONIC EUS
Ultrasound examination of the entire colon can be performed with an ultrasound colonoscope (GF-UM3, CF-UM20, Olympus Corp., Lake Success, NY), which permits direct endoscopic visualization and biopsy in combination with ultrasonographic examination. High-frequency, 20-MHz radial-scanning probes have also been used for staging colorectal tumors (23). Acoustic coupling is again accomplished by infusing water into the lumen or filling with water a latex balloon covering the transducer. In most cases, examination of the cecum is possible. A 5-layer wall architecture can be visualized in the colon wall (1).
Clinical experience with the use of endosonography for staging of more proximal colon cancer is limited. Tio et al. reported their results for 30 patients with colon carcinoma staged with an ultrasound colonoscope (63). Their accuracy in predicting depth of invasion (T stage) was 85% and for lymph-node (N) stage 67%. Most patients with carcinoma of the colon undergo uncomplicated anterior resection to treat symptoms. More prospective evaluations are needed to determine the role of colonic endosonography in local staging of asymptomatic, nonmetastatic colon tumors.
INTEGRATION OF ACCURATE STAGING AND PATIENT MANAGEMENT
The improvement in diagnostic accuracy with EUS over other imaging modalities, and its high correlation with pathologic staging, can aid in the management of colorectal cancer. Reliance on surgery for the accurate staging and possible cure of colorectal cancer without further treatment, along with concerns about about toxicity from combined chemotherapy/radiotherapy, has traditionally resulted in the postoperative use of chemotherapy and/or radiotherapy on the basis of the pathological stage of disease. However, although the benefit of post-operative adjuvant therapy was firmly established in 1991, the perception that its benefit does not outweigh its risks has limited patient acceptance of it. More recently, pre-operative sequencing of chemoradiation therapy has appeared preferable to postoperative treatment because of greater effectiveness and less toxicity (5-22,64-71).
Tumor penetration through the bowel wall and positive nodal status can be predicted through endosonographic evaluation with accuracies of up to 97% and 86%, respectively. These high degrees of accuracy were previously achievable only through surgery plus pathological analysis. Although the level of accuracy will vary with experience, the information obtained with RUS can be used preoperatively to stratify a patient's postoperative risk of tumor recurrence. Patients without metastatic disease, in whom RUS reveals a tumor with a low risk of recurrence, can undergo surgery with a high chance of cure. Patients found at high risk for recurrence despite having resectable disease will benefit from adjuvant radiation and chemotherapy.
Preoperative radiation therapy appears to be better tolerated and associated with less toxicity, and may be biologically more effective than postoperative radiation therapy. Side effects of pelvic radiation therapy are mainly related to small-bowel toxicity (64). Because pelvic surgery may cause the small bowel to fall into the pelvic cavity, a larger volume of small bowel is irradiated if pelvic radiation therapy is given after surgery rather than before. Surgery also causes fibrotic adhesions and fixation that limit bowel motility and expose the bowel to a higher cumulative radiation dose, thus increasing the risk of enteritis. In addition, preoperative radiation therapy may be more biologically effective than postoperative treatment. Because oxygen is a strongly sensitizing agent to radiation (65), radiation given under conditions of good oxygenation may be up to 3 times more tumoricidal than that given under hypoxic conditions such as can occur in a surgically devascularized tumor bed. Another potential advantage to preoperative radiation therapy is sterilization of microscopic disease in the perirectal tissues, which could inhibit tumor-cell seeding from surgical manipulation of a rectal tumor.
In an analysis of randomized trials of adjuvant therapy for rectal cancer, patient compliance was improved if radiotherapy was given before rather than after resection. Compliance was 97% among patients receiving preoperative radiation therapy, as compared to 85% for those receiving postoperative treatment (5). Likewise, acute toxicities were seen in less than 5% of the patients treated preoperatively, as compared to 20% for those given postoperative therapy (5). In a multicenter randomized trial conducted in Sweden, preoperative and postoperative radiation therapy were directly compared (6,7). Preoperatively treated patients received 25.5 Gy in I week. Postoperatively treated patients, if they had stage B2 or C disease, received 60 Gy in 7-8 weeks. Despite the large fraction size utilized in the preoperative group, no significant difference was noted in the incidence of small-bowel obstruction (5%) between this group and patients undergoing surgery alone (6%). In contrast, 11% (p < 0.01) of the patients receiving postoperative radiation developed small-bowel obstruction. Late side effects in the preoperative radiation group were one-half those in the postoperatively treated group.
Preoperative chemoradiotherapy is more effective than surgery alone or surgery plus postoperative chemoradiotherapy. In one study, preoperative irradiation followed by resection resulted in 5-year local control and absolute survival rates of 97% and 66%, respectively, compared with 67% and 40% in a group of patients undergoing surgery alone (8). In 6 randomized studies comparing preoperative radiation therapy plus surgery with surgery alone, local failure in the radiation arms was 35-68% lower than in the arms treated only with surgery (9-14). This compares to a 17-36% reduction in local failure with postoperative radiation versus surgery alone observed in the randomized trials conducted by the Gastrointestinal Tumor Study Group (GITSG) and National Surgical Adjuvant Breast and Bowel Project (NSABP) (66,67). In the prospectively randomized Swedish trial (14) comparing 25.5 Gy preoperative radiation with 60 Gy postoperative radiation, the local recurrence rate was statistically lower in the preoperative arm. Despite the smaller radiation dose given preoperatively, local failure was 12% versus 21% in the postoperative group (p=0.02).
As in the case of radiation therapy, chemotherapy given preoperatively has several potential advantages over postoperative treatment. It appears to be better tolerated and may allow patients to receive higher drug doses (68). Systemic treatment given early, at the time of diagnosis, rather than being delayed until after surgical resection, has the potential to inhibit metastatic spread or combat any distant disease as soon as possible, at a time when the tumor burden is presumably smaller. Certain chemotherapy, such as with 5-fluorouracil (5-FU), can also increase the sensitivity of neoplastic disease to radiation, potentiating the effectiveness of radiation therapy.
RUS permits the selection of patients likely to benefit from preoperative chemoradiation without the need for pathological staging. An advanced unresectable tumor can often be converted by preoperative therapy into a tumor amenable to surgical excision (4). Studies analyzing preoperative chemoradiation for locally advanced rectal cancer show that 90-100% of patients receiving such therapy can subsequently undergo complete resection. Analysis of the resected surgical specimens shows evidence of downstaging in most patients, with a complete pathological response in 10-30% (4,17-22). RUS can reveal whether a tumor is unresectable, and can then be used to follow the progression of downstaging in preoperatively treated patients for evaluation of whether resection becomes feasible (69).
For patients with low-lying rectal cancers, abdomino-perineal resection has been the common surgical approach. Accurate preoperative staging allows alternative sphincter-sparing surgery combined with chemoradiation therapy, or even conservative local excision of the tumor for low-risk patients (70). In a study at Thomas Jefferson University Hospital, patients with tumors in the distal 3 cm of the rectum received 45-60 cGy followed by sphincter-sparing surgery as an alternative to abdomino-perineal resection (15). The 14% local failure rate compares favorably with that among historical controls treated with abdomino-perineal resection. Sphincter function does not appear to be appreciably affected by preoperative radiotherapy. A study at Memorial Sloan-Kettering Cancer Center (MSKCC) in which patients with distal rectal cancer were given preoperative radiation therapy found that more than three-fourths of the patients ultimately rated their sphincter function as being good to excellent (16). Formal assessment of sphincter function by anal manometry found no significant difference in mean maximum squeeze or resting pressure after radiation therapy (71).
Newer surgical techniques for colorectal tumors, such as local excision or laparoscopic resection, are being investigated in appropriate patients. Such surgery is often combined with radiation therapy or chemoradiation as alternatives to radical resection. The operation performed depends on such tumor characteristics as accessibility, size, configuration, ulceration, mobility, depth of invasion, and nodal status. RUS can assume an important role in identifying patients for such surgery. Early results of local excision, with and without chemoradiotherapy, suggest that it is well tolerated; local control of disease is similar to that with more extensive surgery (70). An 89% 5-year survival has been reported for local tumor excision. For similar early T1 or T2 N0 lesions, these results compare very favorably with a 73% 5-year survival following abdominal perineal resection and a 76% 5-year survival following anterior resection (1).
For colorectal. villous adenomas, RUS may become important in screening for invasive malignancy. Hulsmans et al. reported a sensitivity of 75% and specificity of 85% for RUS in detecting malignancy in a colorectal. villous adenoma. (72).
CONCLUSION
With EUS imaging, highly accurate staging of colorectal tumors is now possible without surgery. Precise endosonographic preoperative staging allows use of the TNM pathological staging system to optimally stratify treatment. EUS defines tumor characteristics and allows the selection of patients most likely to benefit from preoperative therapy, or, conversely, the selection of good-risk patients who can safely be treated with surgery alone. EUS allows appropriate preoperative chemoradiation therapy, which appears to be better tolerated and associated with less overall toxicity than postoperative treatment.
In advanced disease, imaging with EUS is unsurpassed for determining the resectability of gastrointestinal neoplasms. In preoperatively treated patients, EUS can be used to monitor the downstaging of tumors prior to their attempted resection. Newer and less invasive surgical techniques allow patients to undergo more limited resections, such as local excision. Criteria are evolving for selecting patients who are candidates for these procedures as alternatives to radical resection.
The clinical utility of EUS for characterizing submucosal masses and evaluating patients for posttreatment recurrence has been demonstrated. When histology is required, EUS has a critical role in defining an abnormal area for deep submucosal biopsy from the gastrointestinal lumen. By significantly improving the diagnostic accuracy of clinical staging, EUS allows the selection for colorectal cancer of treatment that is more effective and produces less morbidity.
REFERENCES
Figure 1. Advanced-stage rectal neoplasm (TNM stage T4 N1 M0). (a) Rectal carcinoma (C) on the anterior wall as seen through the colonoscope with the scope tip at the rectal sphincter. (b) Sonographic image of the carcinoma (C) invading (arrows) through the submucosa (sm), the muscularis propria (mp) and into (T4) the prostate (P). (Tr = scope transducer). (c) Sonographic image of several malignant lymph nodes (In) around the carcinoma (C). (w=wall of rectum; p=prostate)
Figure 2. Sigmoid carcinoma (C) invading through muscularis propria (mp) with associated peritoneal implants (i).